
Since 1963, we have consistently driven product development through scientific research. With 45+ years of continuous accumulation, 3 core patented technologies, and deep collaboration with Japan's top universities, every product has solid scientific evidence.
-
🔬Science-Driven
Product development based on rigorous scientific research, referencing Nobel Prize winner Professor Tasuku Honjo's research field
-
💎Quality First
GMP+ISO dual certification, fully traceable quality management system
-
💡Continuous Innovation
45+ years of continuous R&D investment, 3+ core patented technologies
-
🌏Global Collaboration
Deep collaboration with Japan's top universities, internationally recognized research results
Core Patented Technologies

Using subcritical water at 150-200°C and approximately 10MPa pressure as the extraction solvent, achieving efficient extraction while maintaining the integrity of active ingredients. Compared to traditional high-temperature extraction, active ingredient retention is improved by over 30%, with no chemical solvent residue. Jointly developed with Mie University, Japan.

By activating Tie2 receptors, it regulates vascular endothelial cell function and maintains vascular stability and integrity. Clinical research shows this technology helps promote microcirculation, improve peripheral circulation disorders, and relieve daily discomforts such as cold hands/feet and fatigue.

High-concentration Garcinol patented ingredient extracted from Garcinia. Multiple studies have verified its multiple health support functions including metabolic health support, cellular vitality maintenance, and body balance assistance. Mainly used in CP-101 Kangenriki products.
Using advanced enteric coating technology, capsules can resist gastric acid erosion and precisely release active ingredients in the specific pH environment of the intestine. Achieving targeted absorption, significantly improving bioavailability and maximizing product efficacy.
Research Highlights
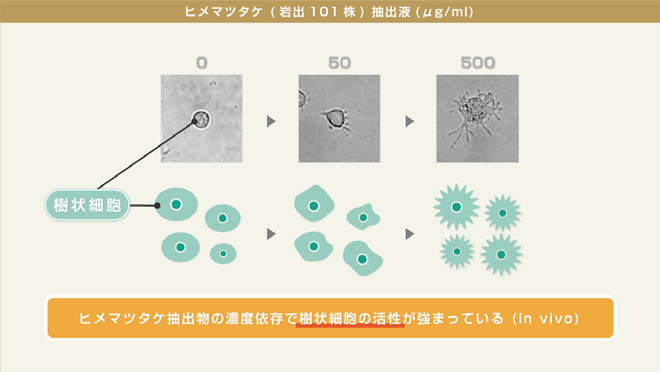
In in vivo and in vitro experiments, CP-101 extract showed positive regulatory effects on immune-related cells. Research indicates the extract promotes specific molecular expression, enhances secretion of health defense-related components, with concentration-dependent effects. Experiments confirmed its contribution to maintaining body balance and health defense.
Clinical research shows that after taking CP-101 Kingo kohonmaru, subjects' skin surface temperature showed positive changes, suggesting potential for promoting smooth circulation. Tie2 pathway activation helps maintain healthy vascular conditions, regulate fluid balance, and relieve daily discomforts like cold hands and feet.
In animal experiments and early research models, continued intake of CP-101 Kangenriki showed positive changes in specific metabolic health-related indicators. Research suggests potential for maintaining healthy cell conditions, supporting overall metabolic capacity, and promoting healthy body shape maintenance.

Subjective feedback from real users shows that after continued use of CP-101 products, most users feel improved daily vitality, reduced fatigue, and better mental state. Some users also reported increased warmth in hands and feet, and improved overall satisfaction.
R&D Strength
-
45+Years of Research
Continuous R&D since 1963
-
3+Core Patents
Exclusive patented technology barriers
-
2+University Partners
Deep collaboration with top universities
-
100%Japanese Quality
Full GMP factory production
Why Choose Japanese Supplement Brands?
Japan's health supplement industry is renowned for strict quality standards and deep R&D heritage
Strict Regulatory Standards
Japan's Pharmaceutical and Medical Device Act and Health Promotion Act impose strict regulations on health supplements, ensuring product safety and efficacy. All products must pass rigorous review before market release.
- Prohibition of false and exaggerated claims
- Strict ingredient labeling requirements
- Regular quality supervision inspections
Deep R&D Heritage
Japanese companies prioritize long-term R&D investment. Many supplement brands have decades of research history. CP-101 has 45 years of Agaricus research experience with 100+ research reports.
- Long-term research data accumulation
- Collaboration with top universities
- Continuous technological innovation
Pharmaceutical-Grade Standards
Japan's GMP certification standards are extremely strict, requiring pharmaceutical-grade production environment and quality control. All CP-101 products are manufactured in GMP·ISO dual-certified facilities.
- Pharmaceutical-grade clean rooms
- Full traceability system
- Third-party testing for each batch
Transparent & Traceable
Japanese brands emphasize ingredient transparency. All ingredients are clearly labeled with traceable origins. CP-101 provides third-party testing reports from SGS and CTI.
- Clear ingredient labeling
- Traceable raw material sources
- Accessible testing reports
CP-101 vs Other Brands: Core Differences
Years R&D History
Japanese Patents
Research Reports
International Certifications
Mie University Collaboration | Subcritical Water Extraction Patent | Tie2 Pathway Patent | GMP·ISO Dual Certification
🌟 Why Choose CP-101: When you choose a Japanese brand, you're choosing not just a product, but decades of R&D heritage, strict quality standards, and a commitment to health. CP-101 represents the high standards of Japan's health supplement industry.
Core Technology Advantages Explained
Subcritical Water Extraction Technology
Patent No: 7141630 | Japanese Invention Patent
This is CP-101's core extraction technology, jointly developed with Mie University, representing advanced technology in Agaricus extraction.
⚙️ Technical Principle
- Uses subcritical water at 150-200°C, ~10MPa pressure
- As extraction solvent
- Special state between gas and liquid
✨ Technical Advantages
- 30%+ higher active ingredient retention
- No chemical solvent residue, safer
- Higher extraction efficiency
- Environmentally friendly
🎯 Application Value
- Preserves β-glucan and other actives
- Enhances product efficacy
- Ensures product safety
💡 Why It Matters: Traditional high-temperature extraction destroys active ingredients, and ordinary solvent extraction may leave chemical residues. Subcritical water extraction perfectly solves both problems—one of the most advanced natural ingredient extraction methods available.
Tie2 Pathway Patent Technology
Patent No: 6246859 | Japanese Invention Patent
This is PRIVITAL's exclusive technology, improving microcirculation by activating Tie2 receptors in vascular endothelium—a revolutionary innovation in men's health.
⚙️ Mechanism of Action
- Long pepper extract activates Tie2 receptors
- Maintains vascular endothelial cell health
- Enhances capillary stability
- Improves peripheral circulation
🎯 Clinical Significance
- Improves cold hands and feet
- Boosts energy and stamina
- Promotes nutrient delivery
- Supports overall circulation
🌟 Exclusive Advantages
- Japanese patent protection
- Scientific research backing
- Targeted, clear effects
- Differentiates from ordinary products
💡 Why It Matters: Ordinary men's supplements rely on hormones or single-nutrient supplementation. Tie2 pathway technology improves microcirculation—a safer, more scientific approach and the fundamental difference between PRIVITAL and other products.
Livinol™ Patented Ingredient
Patent No: 5980228 | US Patent
The core patented ingredient of LIVOSAWA, high-concentration Garcinol extracted from Garcinia, with multiple studies verifying its liver health support.
⚙️ Ingredient Features
- High-concentration Garcinol extraction
- Patented extraction process
- Standardized purity and content
- Good stability
🔬 Research Support
- Multiple in-vitro studies confirm activity
- Animal experiments verify effects
- Safety assessment completed
- Ongoing research follow-up
💪 Mechanism of Action
- Supports normal liver metabolism
- Helps reduce liver burden
- Antioxidant action
- Supports liver health maintenance
💡 Why It Matters: Compared to ordinary milk thistle, Livinol™ has more substantial research evidence and more significant effects. This is the key reason why LIVOSAWA stands out among liver support products.
Ingredient Transparency Commitment
We believe transparency is the foundation of trust
📝 Clear Ingredient Labeling
- All ingredients clearly listed
- Standardized content levels
- No hidden ingredients
- Compliant with food safety regulations
🔍 Traceable Raw Materials
- Complete supplier records
- Batch traceability available
- Full production process documentation
- Comprehensive quality management system
✅ Third-Party Testing
- SGS International Certification
- CTI Testing
- Japan Food Analysis Center
- Testing reports for every batch
🛡️ Our Testing Commitment
Heavy Metals Testing
Lead, mercury, arsenic, cadmium
Pesticide Residue Testing
Meets international standards
Microbial Testing
Bacteria, mold, etc.
Pharmaceutical Screening
Ensuring 100% natural
🔍 Request Testing Reports: Each product has independent testing reports. You can access complete testing data via the QR code on product packaging or contact us.




